Description
- MAXON™ and MAXON™ CV synthetic absorbable sutures are prepared from polyglyconate, a copolymer of glycolic acid and trimethylene carbonate
- MAXON™ and MAXON™ CV synthetic absorbable sutures are indicated for use in general soft tissue approximation and/or ligation, including use in pediatric cardiovascular tissue, where growth is expected to occur, and in peripheral vascular surgery
- MAXON™ and MAXON™ CV synthetic absorbable sutures are not indicated for use in adult cardiovascular tissue, ophthalmic surgery, microsurgery and neural tissues
- MAXON™ sutures retain approximately 75% of initial tensile strength at two weeks, 65% of at three weeks and 50% at four weeks post-implant
- Absorption of MAXON™ sutures is minimal until about 60 days after implant and is essentially complete within six months
- This product is required to be reported under California Proposition 65
| SKU # | 501218 |
| Manufacturer # | SMM-5526 |
| Brand | Maxon™ |
| Manufacturer | Covidien |
| Country of Origin | Unknown |
| Application | Absorbable Suture with Needle |
| Coating | Uncoated |
| Is_Active_Vendor | Y |
| Is_DSCSA | N |
| Is_Discontinued | N |
| Is_Medical_Device | Y |
| Lot_Tracking_Flag | N |
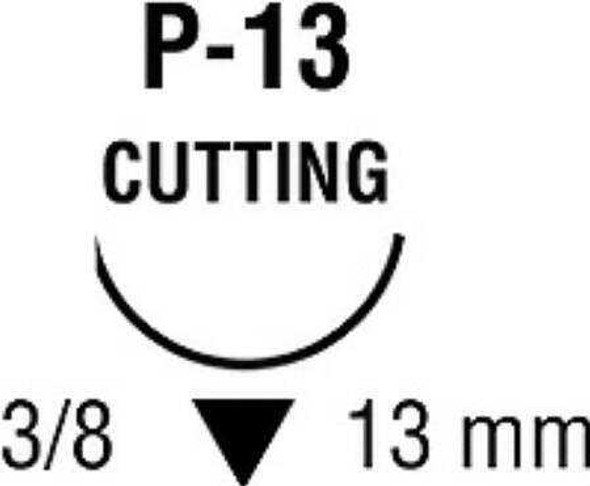
| Needle 1 Code | P-13 |
| Needle Length | 13 mm |
| Needle Length Range | 10.1 to 20 mm |
| Needle Material | Alloy Needle |
| Needle Shape | 3/8 Circle |
| Needle Type | Precision Reverse Cutting Needle |
| Number of Needles | 1-Needle |
| On_Allocation | N |
| Supplier_ID | 487778 |
| Suture Color | Clear |
| Suture Length | 18 Inch Suture |
| Suture Length Range | 10.1 to 20 in |
| Suture Material | Polyglyconate |
| Suture Material Range | Undyed Synthetic |
| Suture Size | Size 5 - 0 |
| Suture Type | Monofilament |
| UNSPSC Code | 42312201 |
Details
SKU: |
501218 |
Brand Name: |
Maxon |
Manufacturer: |
Covidien |
Manufacturer #: |
SMM-5526 |
Country of Origin: |
Unknown |
Application: |
Absorbable Suture with Needle |
Needle 1 Code: |
P-13 |
Needle Length: |
13 mm |
Needle Length Range: |
10.1 to 20 mm |
Needle Material: |
Alloy Needle |
Needle Shape: |
3/8 Circle |
Needle Type: |
Precision Reverse Cutting Needle |
Number of Needles: |
1-Needle |
Suture Color: |
Clear |
Suture Length: |
18'' Suture |
Suture Length Range: |
10.1 to 20 in |
Suture Material: |
Polyglyconate |
Suture Size: |
Size 5 - 0 |
Suture Type: |
P-13 |