Description
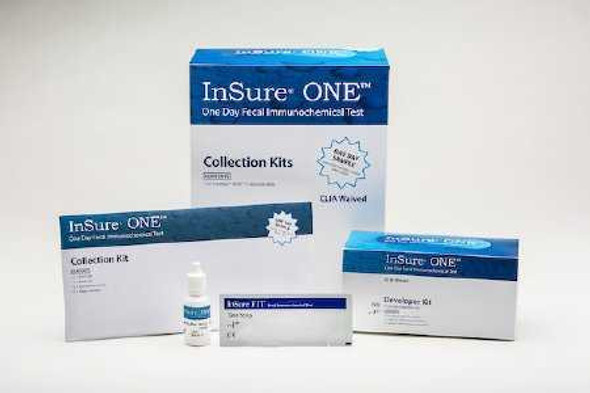
- For development of samples used to detect blood in and around the stool; Developer Kit
- Helps screen for colorectal cancer
- 25 Test Strips included
- Bottles of run buffer supplied with the InSure® ONE™ Developer Kit have been filled to allow slightly more than the 25 test strips supplied to be developed
- The application of additional drops should be avoided and only performed if the test has not developed 5 minutes after the initial application of 8 drops of run buffer
- Does not react with non-human vitamins, drugs or peroxidase from food sources, as InSure ONE is specific for the globin portion of human hemoglobin
| SKU # | 834965 |
| Manufacturer # | 80025.01 |
| Brand | InSure® ONE™ |
| Manufacturer | Enterix |
| Country of Origin | United States |
| Additive | Plain |
| Application | Fecal Specimen Collection Kit |
| Closure Type | Sealable Flap |
| Collection Type | Brush |
| Color | Blue |
| Contents 1 | (25) Test Strips, 9 mL Bottle Run Buffer Reagent |
| Is_Active_Vendor | Y |
| Is_DSCSA | N |
| Is_Discontinued | N |
| Is_Medical_Device | N |
| Lot_Tracking_Flag | N |
| On_Allocation | N |
| Sample Type | Stool Sample |
| Sterility | NonSterile |
| Supplier_ID | 4248618 |
| Type | Fecal Occult Blood Test (iFOB or FIT) |
| UNSPSC Code | 41116168 |
| Volume | 9 mL |