Description
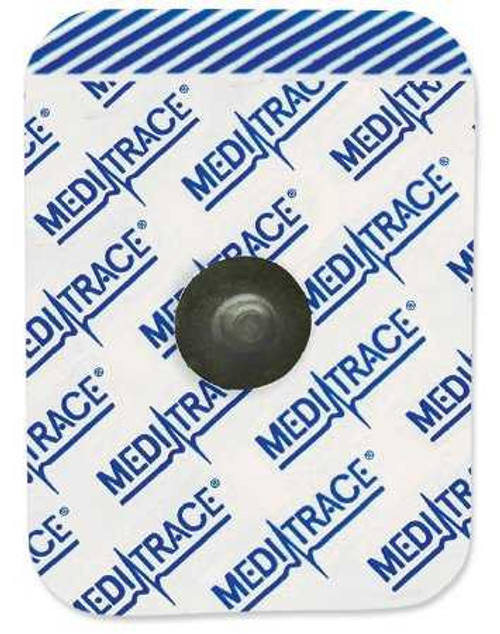
- Kendall™ 930 series radiolucent foam electrodes are the cost-effective solution for general purpose monitoring in high patient turnover areas
- The pre-wired design reduces patient ECG monitoring set-up time making it ideal for emergency departments, surgery centers, and EMS
| SKU # | 1017810 |
| Manufacturer # | 22935 |
| Brand | Medi-Trace® |
| Manufacturer | Cardinal |
| Country of Origin | Canada |
| Application | ECG Monitoring Electrode |
| Backing Material | Foam Backing |
| Connection Type | Snap Connector |
| Gel Type | Conductive Adhesive Gel |
| Is_Active_Vendor | Y |
| Is_DSCSA | N |
| Is_Discontinued | N |
| Is_Medical_Device | Y |
| Lot_Tracking_Flag | N |
| Number per Pack | 5 per Pack |
| On_Allocation | N |
| Securing Method | Aggressive Adhesive |
| Shape | Teardrop |
| Supplier_ID | 488147 |
| UNSPSC Code | 42181701 |
| Usage | Disposable |
| User | Adult |
| X-Ray Compatibility | Radiolucent / MR Tested |