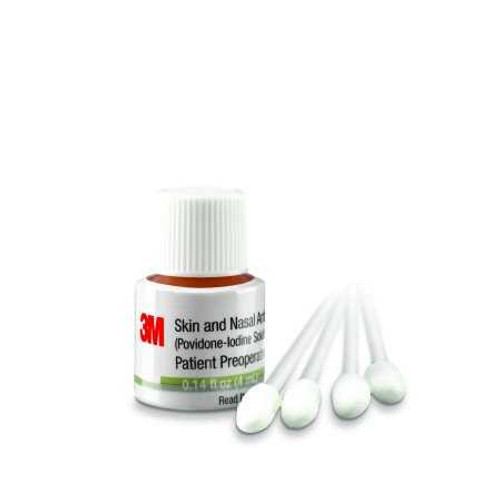
Description
- A pH-balanced formulation with a scientifically developed film-forming polymer to increase persistence
- Helps improve patient safety and protocol compliance without alcohol or antibiotics
- More effective than 10% povidone-iodine for intranasal S. aureus decolonization over the 4 hour time interval
- Evidence-based solution supported by 10 investigator-initiated studies
| SKU # | 739993 |
| Manufacturer # | 192401 |
| Brand | 3M™ |
| Manufacturer | 3M Healthcare US Opco LLC |
| Country of Origin | United States |
| Active Ingredients | Povidone-Iodine |
| Application | Skin and Nasal Antiseptic |
| Container Type | Bottle |
| Form | Liquid |
| Is_Active_Vendor | Y |
| Is_DSCSA | N |
| Is_Discontinued | N |
| Is_Medical_Device | N |
| Lot_Tracking_Flag | N |
| On_Allocation | N |
| Sterility | NonSterile |
| Strength | 5% Strength |
| Supplier_ID | 92585267 |
| UNSPSC Code | 42141500 |
| Volume | 4 mL |