Description
- The single slide design allows convenient testing in batches or as a single specimen submitted to the laboratory
- Each slide has two test areas in order that two sites from each specimen may be tested, increasing accuracy
- WARNING: Cancer and Reproductive Harm - www.P65Warnings.ca.gov
| SKU # | 434306 |
| Manufacturer # | 5072 |
| Brand | ColoScreen® Lab Pack |
| Manufacturer | Helena Laboratories |
| Country of Origin | United States |
| Application | Cancer Screening Test Kit |
| CLIA Classification | CLIA Waived |
| CLIA Classified | CLIA Waived |
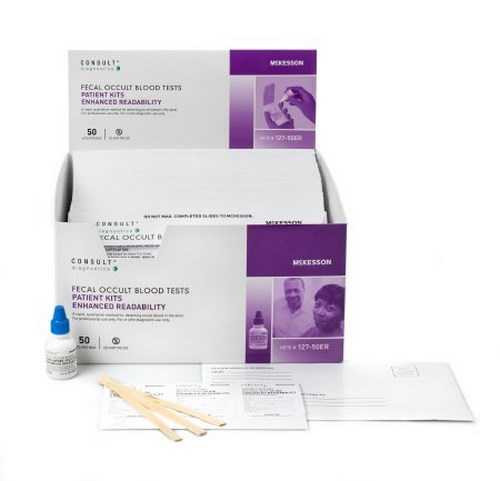
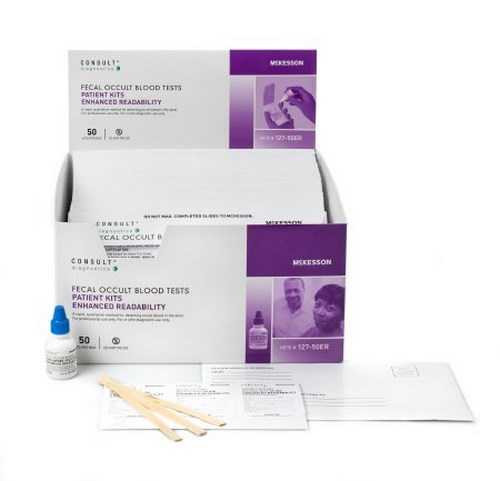
| Contents 1 | (100) Single ColoScreen Slides with On-Slide ColoCheck Monitors, (2) 15 mL ColoScreen Developer, (100) Specimen Applicators |
| Is_Active_Vendor | Y |
| Is_DSCSA | N |
| Is_Discontinued | N |
| Is_Medical_Device | N |
| Lot_Tracking_Flag | N |
| Number of Tests | 100 Tests |
| On_Allocation | N |
| Product Dating | McKesson Acceptable Dating: we will ship >= 90 days |
| Purchase Program Type | Standard Purchase |
| Reading Type | Visual Read |
| Sample Type | Stool Sample |
| Specialty | Chemistry |
| Supplier_ID | 488163 |
| Technology | Guaiac |
| Test Format | Single Slide Format |
| Test Kit Type | Rapid |
| Test Name | Fecal Occult Blood Test (FOBT) |
| Test Type | Colorectal Cancer Screening |
| Time to Results | 2 Minute Results |
| UNSPSC Code | 41116129 |