Description
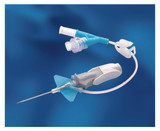
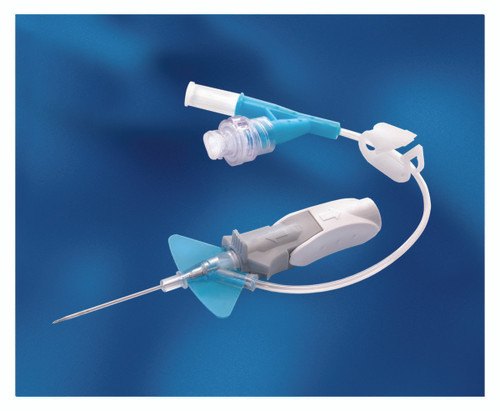
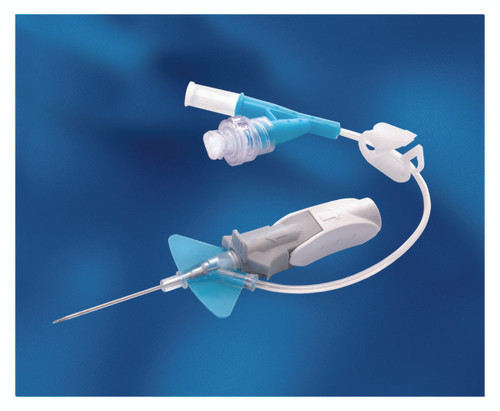
- The catheter is integrated with a soft, flexible stabilization platform designed for stability, comfort, and to minimize catheter movement in the vessel, as well as irritation to the vessel
- Instaflash Needle Technology is designed to reduce hit-and-miss insertion by confirming the vessel entry, enabling you to stay focused on the insertion site
- Catheter 0.36 ID and 0.71 OD
- As you withdraw the needle, passive needle-shielding technology is engaged
Product Details
- Application:
- Closed IV Catheter
- Manufacturer Name:
- BD
- Manufacturer Number:
- 383531
- QTY Per Sell:
- 80
- Sterility:
- Sterile
- Usage:
- Disposable
- Catheter Length:
- 3/4 Inch
- Catheter Material:
- Vialon Biomaterial
- Color Code:
- Yellow
- Flow Rate:
- 18 mL / min
- Gauge:
- 24 Gauge
- Hub Material:
- Plastic Hub
- Hub Type:
- Winged Hub
- Needle Material:
- Stainless Steel
- Number of Ports:
- Dual Port
- Port Type:
- Needleless Connector
- Safety Feature:
- Sliding
- Tubing Diameter:
- 1.2 mm ID
- X-Ray Compatibility:
- Radiopaque
- Is New (Last 30):
- No
- Legend Code:
- Yes
- Country of Origin:
- Singapore