Description
- McKesson Surgical Opthalmic Drape
- Delivers optimal protection and performance in creating and maintaining a sterile field during ophthalmic procedures
- Made of medical-grade embossed PE film for quality softness and drapeability
- Translucent material allows physician unobstructed view of surgery area
- Full incise fenestration
- Quality 3M®-brand film adhesive provides strong adhesion during use for added security and to reduced risk of skin irritation or injury upon removal
- Adhesive film isolates the surgical site from eye lid margins, eyelashes and eyebrow to help reduce the risk of surgical site contamination
- Included fluid collection pouch provides convenience in controlling excess fluid
- Low-flammability and low-linting features for to reduced procedural risks
- ASTM 1670
- Meets all industry-required ASTM and ISO standards
- Meets standard for ISO 10993 Biocompatibility Testing for Cytotoxicity, Irritation, and Sensitization
- Individually packaged, disposable design for single sterile use and consistent quality
- Not Made with Natural Rubber Latex
- Packaged: 1 Per Pack, 30 Packs Per Case
| SKU # | 1101301 |
| Manufacturer # | 183-I80-08129-S |
| Brand | McKesson |
| Manufacturer | McKesson Brand |
| Country of Origin | China |
| Application | EENT Drape |
| Color | Clear |
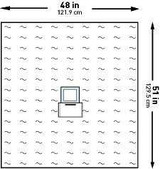
| Dimensions | 48 W X 51 L Inch |
| Drape Name | Surgical Ophthalmic Drape |
| Extremity Cover | Without Covers |
| Fluid Collection Type | Fluid Collection Pouch |
| Instrument Holders | Without Instrument Holder |
| Is_Active_Vendor | Y |
| Is_DSCSA | N |
| Is_Discontinued | N |
| Is_Medical_Device | N |
| Lot_Tracking_Flag | N |
| Material | Embossed PE Film |
| On_Allocation | N |
| Reinforcement Type | Without Reinforcement |
| Securing Method | Adhesive Fenestration |
| Sterility | Sterile |
| Supplier_ID | 55556118 |
| Surgical Area | Fenestrated |
| Surgical Area Specifications | Incise Area 3-7/8 X 4-7/8 Inch |
| Tube and Cord Holders | Without Cord and Tube Holders |
| UNSPSC Code | 42131701 |
| Latex Free Indicator | Not Made with Natural Rubber Latex |
Product Details
- Application:
- EENT Drape
- Color:
- Clear
- Dimensions:
- 48 W X 51 L Inch
- Drape Name:
- Surgical Ophthalmic Drape
- Extremity Cover:
- Without Covers
- Fluid Collection Type:
- Fluid Collection Pouch
- Instrument Holders:
- Without Instrument Holder
- Latex Free Indicator:
- Not Made with Natural Rubber Latex
- Manufacturer Number:
- 183-I80-08129-S
- Manufacturer Name:
- McKesson MedSurg
- Material:
- Embossed PE Film
- Reinforcement Type:
- Without Reinforcement
- Securing Method:
- Adhesive Fenestration
- Sterility:
- Sterile
- Surgical Area:
- Nonfenestrated
- Surgical Area Specifications:
- Incise Area 3-7/8 X 4-7/8 Inch
- Tube and Cord Holders:
- Without Cord and Tube Holders
- UNSPSC Code:
- 42131701