Description
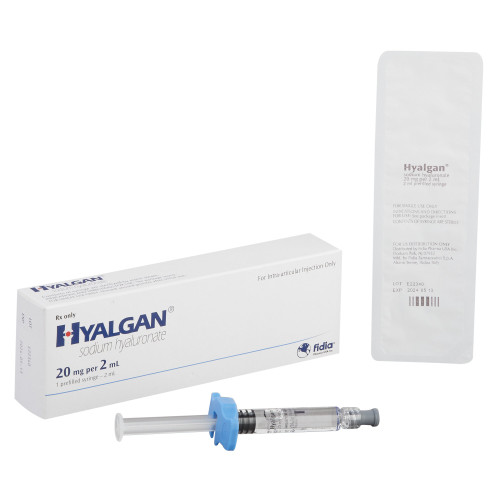
- Hyalgan® is indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative nonpharmacologic therapy, and to simple analgesics
Product Details
- Application:
- Osteoarthritis Agent
- Container Type:
- Prefilled Syringe
- Dosage Form:
- Injection
- Generic Drug Code:
- 32121
- Generic Drug Name:
- Sodium Hyaluronate
- Manufacturer Number:
- 89122072420
- Manufacturer Name:
- Fidia Pharma USA
- NDC Number:
- 89122072420
- Product Dating:
- McKesson Acceptable Dating: we will ship >= 180 days
- Strength:
- 20 mg / mL
- Type:
- Intra-articular
- UNSPSC Code:
- 51202002
- Volume:
- 2 mL