Description
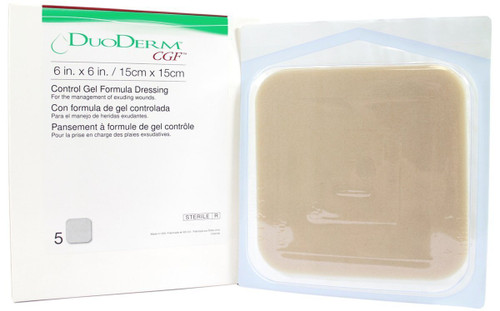
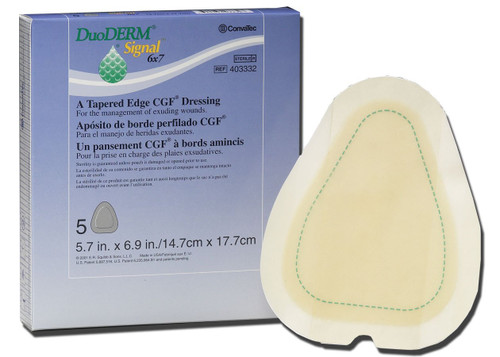
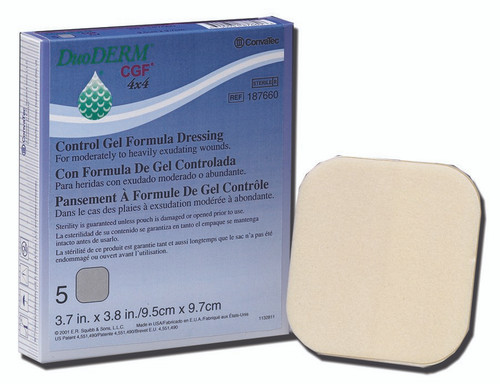
- This 4 × 5 inch triangle DuoDerm CGF border dressing consists of a foam layer and a skin contact layer with a hydrocolloid formulation for maximum effectiveness.
- Apply it to areas that are difficult to dress.
- The non-adhesive dressing gives users protection from bacteria and viruses such as HBV and HIV.
- The wide outer foam border eliminates the need for supplemental taping.
Product Details
- Application:
- Hydrocolloid Dressing
- Manufacturer Name:
- ConvaTec
- Manufacturer Number:
- 187973
- Backing Material:
- Foam Backing
- Color:
- Beige
- Dimensions:
- 4 X 5 Inch
- Length:
- 5 to 12 Inch Length
- Material:
- Hydrocolloid
- QTY Per Sell:
- 5
- Shape:
- Triangle
- Sterility:
- Sterile
- Is New (Last 30):
- No
- California Prop 65:
- No
- Legend Code:
- No
- FSA Eligible:
- Yes
- UPC:
- 300031879737
- Country of Origin:
- Dominican Republic
- Item Allocation:
- N