Description
- McKesson Under Buttocks Drape
- Delivers optimal protection and performance in creating and maintaining a sterile field during surgery
- For use in perineal and gynecological procedures
- Impervious Pe film prevents fluid strike-through to avoid contaminating bedding and equipment
- Specifically designed SMS fabric reinforcement provides fluid repellency, added durability and patient comfort
- Low-linting fabric properties reduce particle generation - a known source of post-operative complications caused by bacterial transmission or foreign body contamination
- Flame-resistant materials prevent ignition risks associated with the use of high-energy medical devices during surgery
- Includes fluid collection pouch to contain fluids and provides easy cleanup and disposal for better sterility maintece and less mess
- Attached fluid collection pouch designed with malleable band to promote efficient fluid control
- Designed with clear instructional stamps to promote ease of use, aseptic presentation, and intuitive drape placement
- Individually packaged, disposable design for single sterile use and consistent quality
- Meets standard for ASTM D5034-2009 for Breaking Strength and Elongation of Textile Fabrics (Grab Test)
- Meets Class 1 Standard for Flammability of Clothing Textiles 16 CFR Part 1617
- Meets standard for ISO 9073-10-2003 Textiles, Part 10 for Lint-Resistance
- Meets standard for ISO 10993 Biocompatibility Testing for Cytotoxicity, Irritation, and Sensitization
- Meets standard for ISO 12945-2 abrasion resistance
- Not Made with Natural Rubber Latex
- Packaged: 1 Per Pack, 25 Packs Per Case
| SKU # | 1101265 |
| Manufacturer # | 183-I80-14112-S |
| Brand | McKesson |
| Manufacturer | McKesson Brand |
| Country of Origin | China |
| Application | Obstetrics / Gynecology Drape |
| Color | Blue |
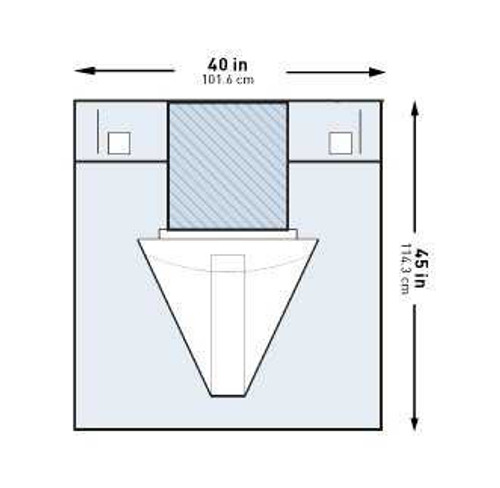
| Dimensions | 40 W X 45 L Inch |
| Drape Name | Under Buttocks Drape |
| Extremity Cover | Without Covers |
| Fluid Collection Type | Fluid Collection Pouch |
| Instrument Holders | Without Instrument Holder |
| Is_Active_Vendor | Y |
| Is_DSCSA | N |
| Is_Discontinued | N |
| Is_Medical_Device | N |
| Lot_Tracking_Flag | N |
| Material | PE Blue Film |
| On_Allocation | N |
| Pouch Specifications | N/A |
| Reinforcement Type | SMS Fabric Reinforcement |
| Securing Method | Without Adhesive |
| Sterility | Sterile |
| Supplier_ID | 55556118 |
| Surgical Area | Nonfenestrated |
| Tube and Cord Holders | Without Cord and Tube Holders |
| UNSPSC Code | 42131701 |
| Latex Free Indicator | Not Made with Natural Rubber Latex |
Product Details
- Application:
- Obstetrics / Gynecology Drape
- Color:
- Blue
- Dimensions:
- 40 W X 45 L Inch
- Drape Name:
- Under Buttocks Drape
- Extremity Cover:
- Without Covers
- Fluid Collection Type:
- Fluid Collection Pouch
- Instrument Holders:
- Without Instrument Holder
- Latex Free Indicator:
- Not Made with Natural Rubber Latex
- Manufacturer Number:
- 183-I80-14112-S
- Manufacturer Name:
- McKesson MedSurg
- Material:
- PE Blue Film
- Pouch Specifications:
- N/A
- Reinforcement Type:
- SMS Fabric Reinforcement
- Securing Method:
- Without Adhesive
- Sterility:
- Sterile
- Surgical Area:
- Nonfenestrated
- Tube and Cord Holders:
- Without Cord and Tube Holders
- UNSPSC Code:
- 42131701