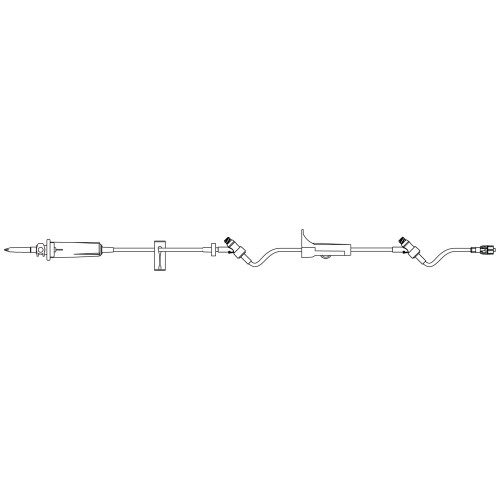
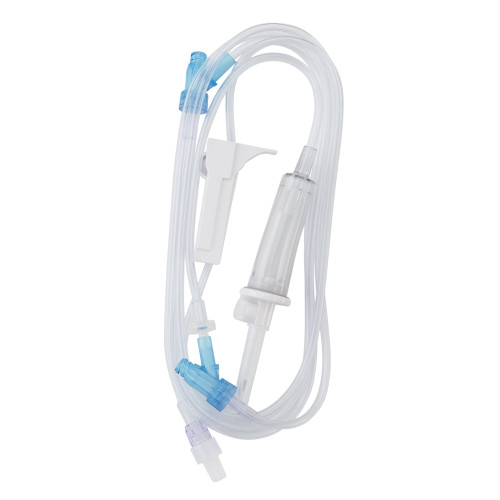
Description
- Three SafeDAY Injection Sites for enhanced safety and convenience
- Transparent tubing for easy monitoring of fluid flow
- Latex-free materials to reduce the risk of allergic reactions
- Universal spike for compatibility with a wide range of IV bags
- Roller clamp for precise fluid flow control
- Needle-free access ports to minimize the risk of needlestick injuries
- DEHP-free construction for a safer, more environmentally friendly option
Product Details
- Application:
- Secondary Administration Set
- Manufacturer Name:
- B. Braun
- Manufacturer Number:
- 352640
- QTY Per Sell:
- 50
- Sterility:
- Sterile
- Type:
- Solution
- Administration Method:
- Gravity
- Drip Rate:
- 15 Drops / mL Drip Rate
- Flow Control Type:
- Roller Clamp / Slide Clamp
- Number of Ports:
- 3 Ports
- Port Type:
- SafeDayª Valve Port
- Priming Volume:
- 18.9 mL Priming Volume
- Tubing Length:
- 112 Inch Tubing
- Is New (Last 30):
- No
- Legend Code:
- Yes