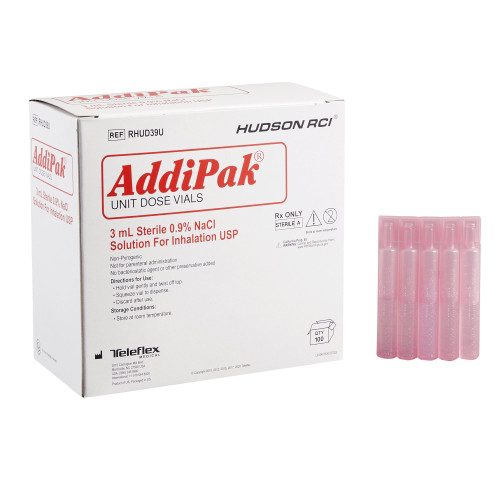
Description
- Nebulizer Vial
- Individually foil pouched and embossed vials for easy identification
- Preservative and additive free
- For respiratory therapy, not for parenteral administration
Product Details
- Application:
- Saline Solution
- Manufacturer Name:
- Nephron Pharmaceutical
- Manufacturer Number:
- 00487930133
- Container Type:
- Unit Dose Vial
- Dosage Form:
- Solution
- QTY Per Sell:
- 30
- Strength:
- 0.9%
- Type:
- Inhalation
- Volume:
- 3 mL
- Is New (Last 30):
- No
- Legend Code:
- Yes
- Country of Origin:
- Unknown