Description
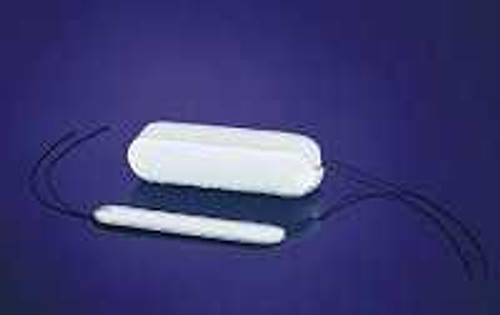
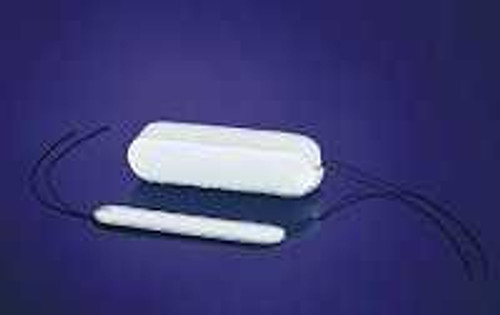
- PVA expandacell foam for epistaxis management after trauma or surgery
- Patented applicator protects foam from moisture prior to insertions, aids in placement and insertion, minimizes blood containment concerns, 30 seconds to insert
- Provides gentle, but firm mucosal compression on the tamponade of anterior or middle segment epistaxis
- When in contact with moisture, foam absorbs and swells to six times its compressed diameter
- String attached for retention and easy removal
- String attached for retention and easy removal
| SKU # | 383202 |
| Manufacturer # | 11S-S0300-08AS |
| Brand | Rhino Rocket® |
| Manufacturer | Summit Medical |
| Country of Origin | Unknown |
| Application | Nasal Packing with Applicator |
| Container Type | Pack |
| Dimensions | 1 X 3 X 3 cm |
| Impregnated Ingredients | Non-impregnated |
| Is_Active_Vendor | Y |
| Is_DSCSA | N |
| Is_Discontinued | N |
| Is_Medical_Device | N |
| Lot_Tracking_Flag | N |
| Material | PVA Expandacell® Foam |
| On_Allocation | N |
| Sterility | Sterile |
| Supplier_ID | 3885348 |
| UNSPSC Code | 42312402 |
Product Details
- Application:
- Nasal Packing
- Container Type:
- Pack
- Dimensions:
- 1 X 3 X 3 cm
- Impregnated Ingredients:
- Non-impregnated
- Manufacturer Number:
- 11S-S0300-08AS
- Manufacturer Name:
- Summit Medical L
- Material:
- PVA Expandacell® Foam
- Sterility:
- Sterile
- UNSPSC Code:
- 42312402
- Volume:
- 8 Count