Description
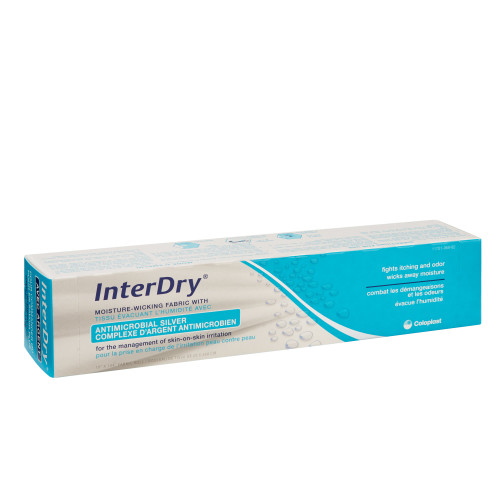
- Water-based gel wound dressing contains silver that in laboratory tests has been shown to inhibit the growth of microorganisms such as: Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, antibiotic resistant strains, MRSA and VRE, as well as fungi such as Candida albicans and Aspergillus niger
- 3-ounce tube
Product Details
- Application:
- Silver Dressing
- Manufacturer Name:
- ABL Medical
- Manufacturer Number:
- 31060000663
- Material:
- Gel
- QTY Per Sell:
- 1
- Sterility:
- NonSterile
- Volume:
- 3 oz.
- Is New (Last 30):
- No
- California Prop 65:
- No
- Legend Code:
- Yes
- UPC:
- 831060006639
- Country of Origin:
- Unknown