Description
- Designed with continuous broad-spectrum antimicrobial activity in the drape adhesive where it can't be washed away
- Clinically shown to help reduce the risk of wound contamination and immobilize bacteria on the skin1,2
- Drape and liner feature a full-width handle for control of liner release and drape application
- Strip is imprinted with stop signs to indicate when the end of the drape is near
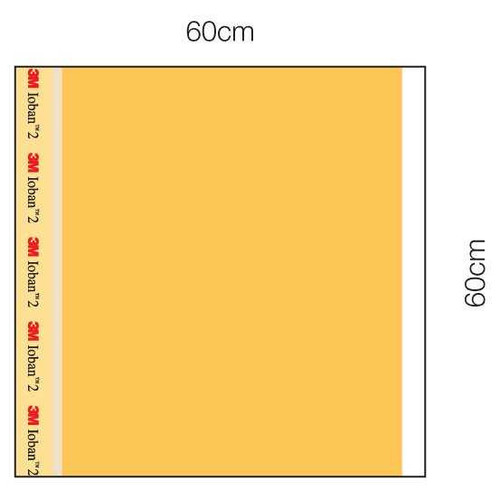
- Surgical drapes with an antimicrobial film designed to provide a sterile surface all the way to the wound edge and continuous antimicrobial activity throughout the procedure
- Incise drapes are made with a "low memory" film that conforms to body contours and stretches to allow limb manipulation
| SKU # | 405682 |
| Manufacturer # | 6648EZ |
| Brand | 3M™Ioban™2 |
| Manufacturer | 3M Healthcare US Opco LLC |
| Country of Origin | United States |
| Application | Surgical Drape |
| Color | Yellow |
| Dimensions | 23 W X 23 L Inch |
| Drape Name | Antimicrobial Incise Drape |
| Extremity Cover | Without Covers |
| Fluid Collection Type | Without Pouch |
| Instrument Holders | Without Instrument Holder |
| Is_Active_Vendor | Y |
| Is_DSCSA | N |
| Is_Discontinued | N |
| Is_Medical_Device | N |
| Lot_Tracking_Flag | N |
| Material | Ioban™ 2 Incise Film |
| On_Allocation | N |
| Reinforcement Type | Without Reinforcement |
| Securing Method | Without Adhesive |
| Sterility | Sterile |
| Supplier_ID | 92585267 |
| Surgical Area | Nonfenestrated |
| Surgical Area Specifications | Incise Area 23 X 23 Inch, Antimicrobial |
| Tube and Cord Holders | Without Cord and Tube Holders |
| UNSPSC Code | 42131701 |
| Latex Free Indicator | Not Made with Natural Rubber Latex |
Product Details
- Application:
- Surgical Drape
- Color:
- Yellow
- Dimensions:
- 23 W X 23 L Inch
- Drape Name:
- Antimicrobial Incise Drape
- Extremity Cover:
- Without Covers
- Fluid Collection Type:
- Without Pouch
- Instrument Holders:
- Without Instrument Holder
- Latex Free Indicator:
- Not Made with Natural Rubber Latex
- Material:
- Ioban™ 2 Incise Film
- Reinforcement Type:
- Without Reinforcement
- Securing Method:
- Without Adhesive
- Sterility:
- Sterile
- Surgical Area:
- Nonfenestrated
- Surgical Area Specifications:
- Incise Area 23 X 23 Inch, Antimicrobial
- Tube and Cord Holders:
- Without Cord and Tube Holders
- UNSPSC Code:
- 42131701