Description
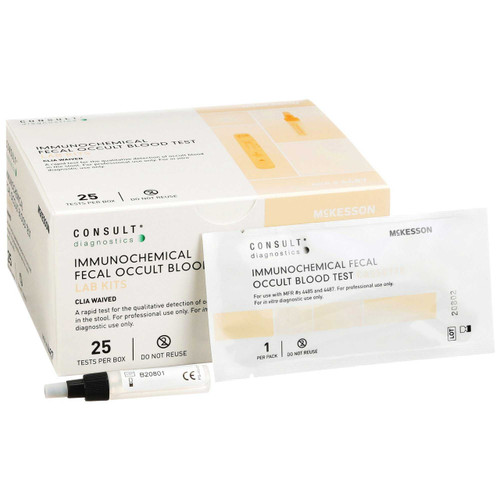
- Consult™ iFOBT Cassette Kit
- CLIA-waived
- Contents: 25 Cassettes, 25 Preparation Buffer Tubes, Instructional Insert
- Not Made with Natural Rubber Latex
- Packaged: 25 Tests Per Box
| SKU # | 854860 |
| Manufacturer # | 4487 |
| Brand | Consult™ |
| Manufacturer | McKesson Brand |
| Country of Origin | United States |
| Application | Cancer Screening Test Kit |
| CLIA Classification | CLIA Waived |
| CLIA Classified | CLIA Waived |
| Contents 1 | (25) Cassettes, (25) Preparation Buffer Tubes, Instructional Insert |
| HCPCS | G0328 (Disclaimer) |
| Is_Active_Vendor | Y |
| Is_DSCSA | N |
| Is_Discontinued | N |
| Is_Medical_Device | N |
| Lot_Tracking_Flag | N |
| Number of Tests | 25 Tests |
| On_Allocation | N |
| Product Dating | McKesson Acceptable Dating: we will ship >= 180 days |
| Purchase Program Type | Standard Purchase |
| Reading Type | Visual Read |
| Sample Type | Stool Sample |
| Specialty | Immunoassay |
| Supplier_ID | 4442142 |
| Test Format | Cassette Format |
| Test Kit Type | Rapid |
| Test Name | Fecal Occult Blood Test (iFOB or FIT) |
| Test Type | Colorectal Cancer Screening |
| Time to Results | 5 Minute Results |
| UNSPSC Code | 41116126 |
| Latex Free Indicator | Not Made with Natural Rubber Latex |
Product Details
- Application:
- Cancer Screening Test Kit
- Contents:
- (25) Cassettes, (25) Preparation Buffer Tubes, Instructional Insert
- Latex Free Indicator:
- Not Made with Natural Rubber Latex
- QTY Per Sell:
- 25
- Number of Tests:
- 25 Tests
- Reading Type:
- Visual Read
- Sample Type:
- Stool Sample
- Is New (Last 30):
- No
- California Prop 65:
- No
- Legend Code:
- Yes
- Country of Origin:
- United States
- Item Allocation:
- N