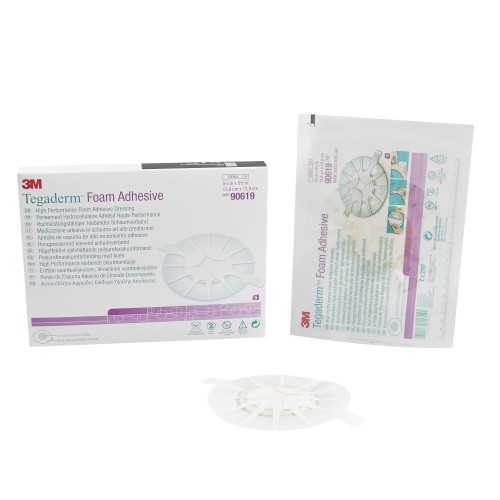
Description
- Multi-layer design provides high absorbency with high breathability to reduce the risk of maceration
- Adapts to changing levels of exudate to maintain moisture balance for optimal wound healing
- Particularly suited for situations where increased securement or longer wear time is needed
- Conformable for difficult body contours
- 3 x 3 inch pad
Product Details
- Application:
- Foam Dressing
- Manufacturer Name:
- 3M
- Manufacturer Number:
- 90619
- Adhesive Type:
- Acrylic Adhesive
- Backing Material:
- Film Backing
- Color:
- White
- Dimensions:
- 5-1/2 X 5-1/2 Inch
- Latex Free Indicator:
- Not Made with Natural Rubber Latex
- Length:
- 5 to 12 Inch Length
- Material:
- Polyurethane Foam
- Pad Size:
- 3 X 3 Inch Pad
- QTY Per Sell:
- 20
- Shape:
- Heel
- Sterility:
- Sterile
- Is New (Last 30):
- No
- California Prop 65:
- No
- Legend Code:
- No
- Country of Origin:
- Unknown